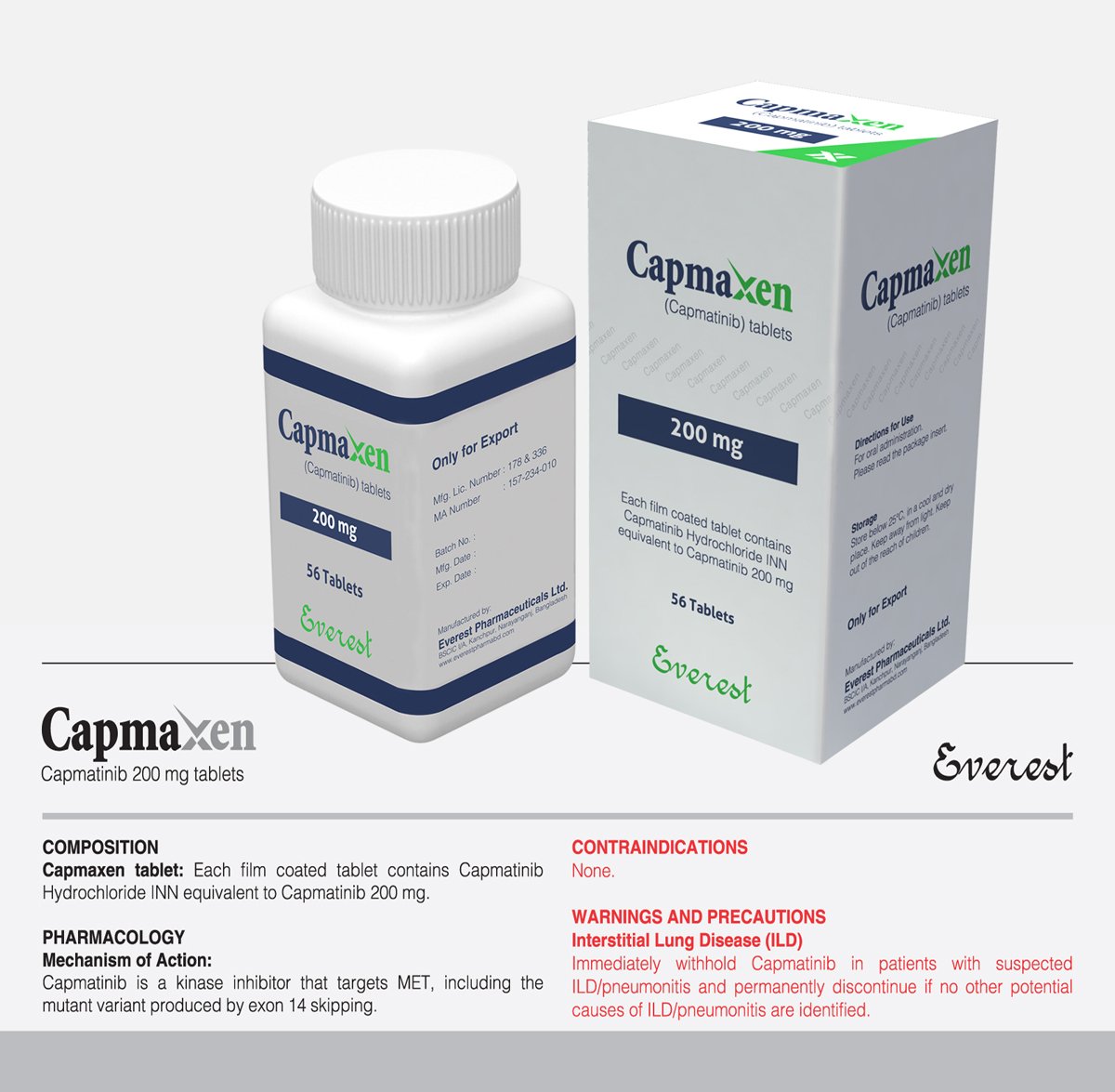
Capmaxen (Capmatinib INN) 200 mg
£0.00
- Patients should be vigilant for signs and symptoms of interstitial lung disease/pneumonitis, with discontinuation of treatment if severe symptoms manifest.
- Regular monitoring of liver function tests is recommended during Capmatinib therapy.
- Electrocardiograms (ECGs) are advised before initiation and periodically during treatment to monitor for QT interval prolongation.
- Due to the risk of embryo-fetal toxicity, effective contraception is advised for women of childbearing potential during Capmatinib treatment and for at least one week after the final dose.
Indication: Capmatinib, under the International Nonproprietary Name (INN) Capmatinib, is prescribed for treating metastatic non-small cell lung cancer (NSCLC) in adults with specific mutations in the MET gene.
Pharmacology: Capmatinib acts as a potent and selective inhibitor of the MET receptor tyrosine kinase. By binding to the MET receptor, it blocks its activation and downstream signaling pathways, effectively inhibiting tumor cell growth, survival, and spread.
Dosage and Administration: Capmatinib is typically administered orally at a dose of 400 mg twice daily, with or without food. Treatment should continue until either disease progression or intolerable adverse effects emerge.
Interactions: Potential interactions may occur with medications that affect CYP3A4 and CYP2C8 enzymes. It is advisable to avoid combining Capmatinib with potent CYP3A4 inhibitors or inducers. Caution is warranted when co-administering drugs that prolong the QT interval or substrates of P-glycoprotein.
Side Effects: Common side effects of Capmatinib comprise peripheral edema, nausea, vomiting, fatigue, reduced appetite, diarrhea, and elevated liver enzymes. Serious adverse reactions may include interstitial lung disease/pneumonitis, liver toxicity, and QT interval prolongation.
Precautions and Warnings:
Overdose Effect: In cases of overdose, supportive care is essential. No specific antidote for Capmatinib overdose exists, necessitating close monitoring for adverse reactions and prompt initiation of appropriate symptomatic treatment as required.
| Product Name | Capmaxen |
|---|---|
| Generic Name | Capmatinib INN |
| Formulation | Tablet |
| Available Pack Size | 56 Tablets |
| Available Strength | 200 mg |
You must be logged in to post a review.



Reviews
There are no reviews yet.