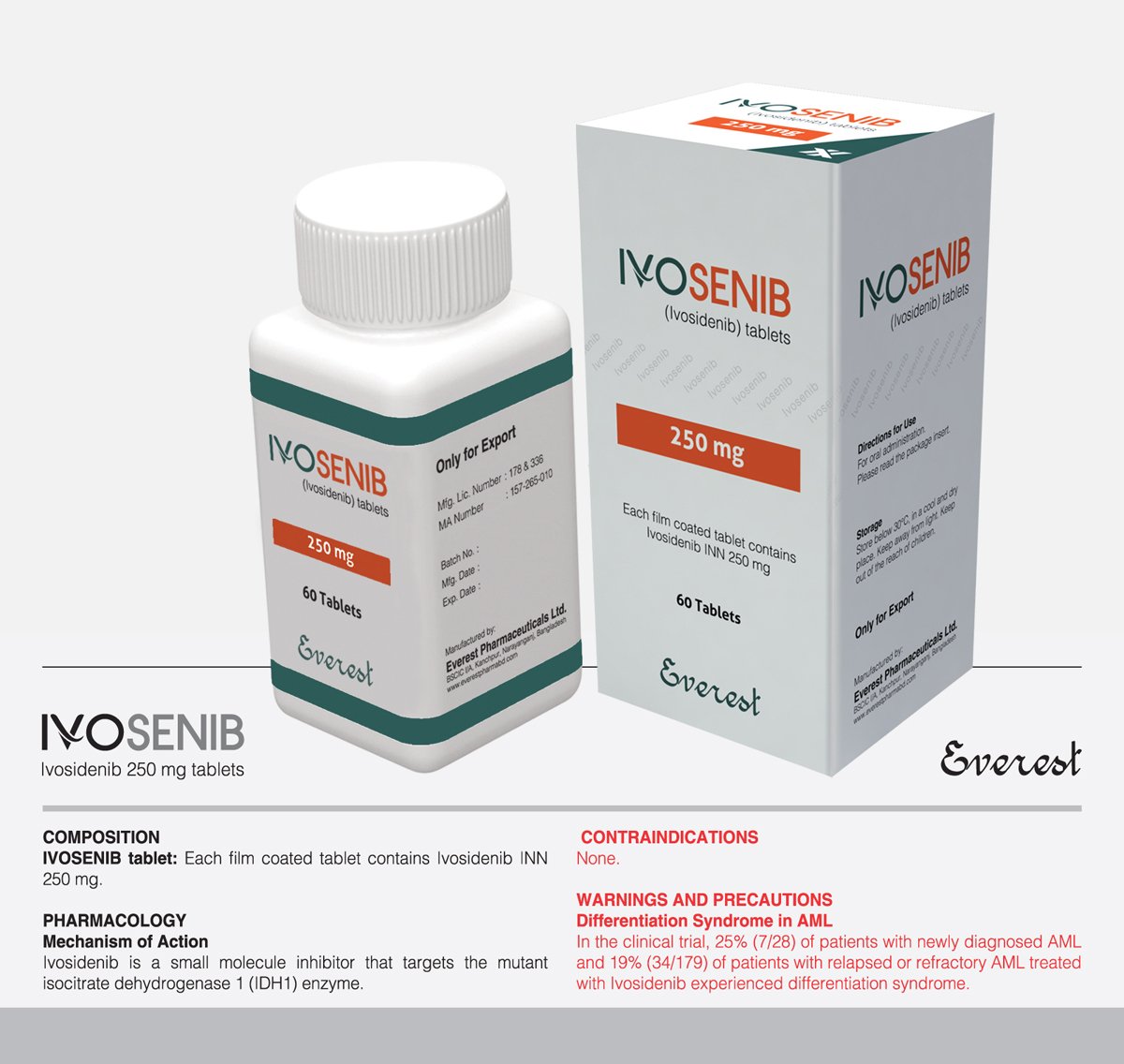
Ivosenib (Ivosidenib INN) 250 mg
£0.00
Ivosenib, also known as Ivosidenib, is indicated for adults with relapsed or refractory acute myeloid leukemia harboring an isocitrate dehydrogenase-1 (IDH1) mutation. Acting as a selective inhibitor of mutant IDH1 enzymes, Ivosenib restores normal cellular differentiation by blocking abnormal enzyme activity. Administered orally at 500 mg once daily, this treatment should be continued until disease progression or intolerable side effects occur. While generally well-tolerated, common side effects include fatigue, nausea, and diarrhea, with precautions advised for potential adverse reactions such as differentiation syndrome and hepatotoxicity. Overdose management involves supportive care, highlighting the importance of close monitoring during treatment.
- Patients should undergo regular monitoring for signs indicative of differentiation syndrome, such as fever, dyspnea, and acute respiratory distress. Immediate treatment initiation is crucial if differentiation syndrome is suspected.
- Regular liver function tests are recommended throughout Ivosenib treatment to monitor for hepatotoxicity.
- Periodic QT interval monitoring is advised, especially in patients with a history of QT prolongation or those taking medications known to prolong the QT interval.
- Due to the potential for embryo-fetal toxicity, effective contraception is advised during Ivosenib treatment and for at least one month following the last dose.
Indication: Ivosenib, also identified as Ivosidenib according to the International Nonproprietary Name (INN), is prescribed for adults diagnosed with relapsed or refractory acute myeloid leukemia (AML) featuring a susceptible isocitrate dehydrogenase-1 (IDH1) mutation, confirmed by an FDA-approved test.
Pharmacology: Functioning as a selective inhibitor of mutant IDH1 enzymes, particularly targeting the R132 mutation, Ivosenib obstructs the abnormal activity of mutant IDH1 enzymes. This action aids in restoring normal cellular differentiation and diminishing the production of 2-hydroxyglutarate (2-HG), a metabolite associated with the development of leukemia.
Dosage and Administration: Patients are typically advised to take Ivosenib orally at a recommended dosage of 500 mg once daily, irrespective of food intake. Tablets should be swallowed whole without crushing or splitting. Treatment is generally continued until disease progression or the emergence of unacceptable toxicity.
Interactions: Ivosenib has the potential to interact with medications influencing cytochrome P450 enzymes, particularly CYP3A4 and CYP2C9. Co-administration with potent CYP3A4 inhibitors or inducers should be approached cautiously. Vigilant monitoring is essential when combining Ivosenib with other drugs.
Side Effects: Frequent side effects associated with Ivosenib include fatigue, nausea, diarrhea, leukocytosis, QT prolongation, and differentiation syndrome. Serious adverse reactions may encompass IDH differentiation syndrome, leukocytosis, and posterior reversible encephalopathy syndrome (PRES).
Precautions and Warnings:
Overdose Effect: In the event of Ivosenib overdose, supportive care is essential. Specific antidotes are unavailable; therefore, close monitoring for adverse reactions is vital, with symptomatic treatment initiated as necessary.
| Product Name | Ivosenib |
|---|---|
| Generic Name | Ivosidenib INN |
| Formulation | Tablet |
| Available Pack Size | 60 Tablets |
| Available Strength | 250 mg |
You must be logged in to post a review.
Reviews
There are no reviews yet.