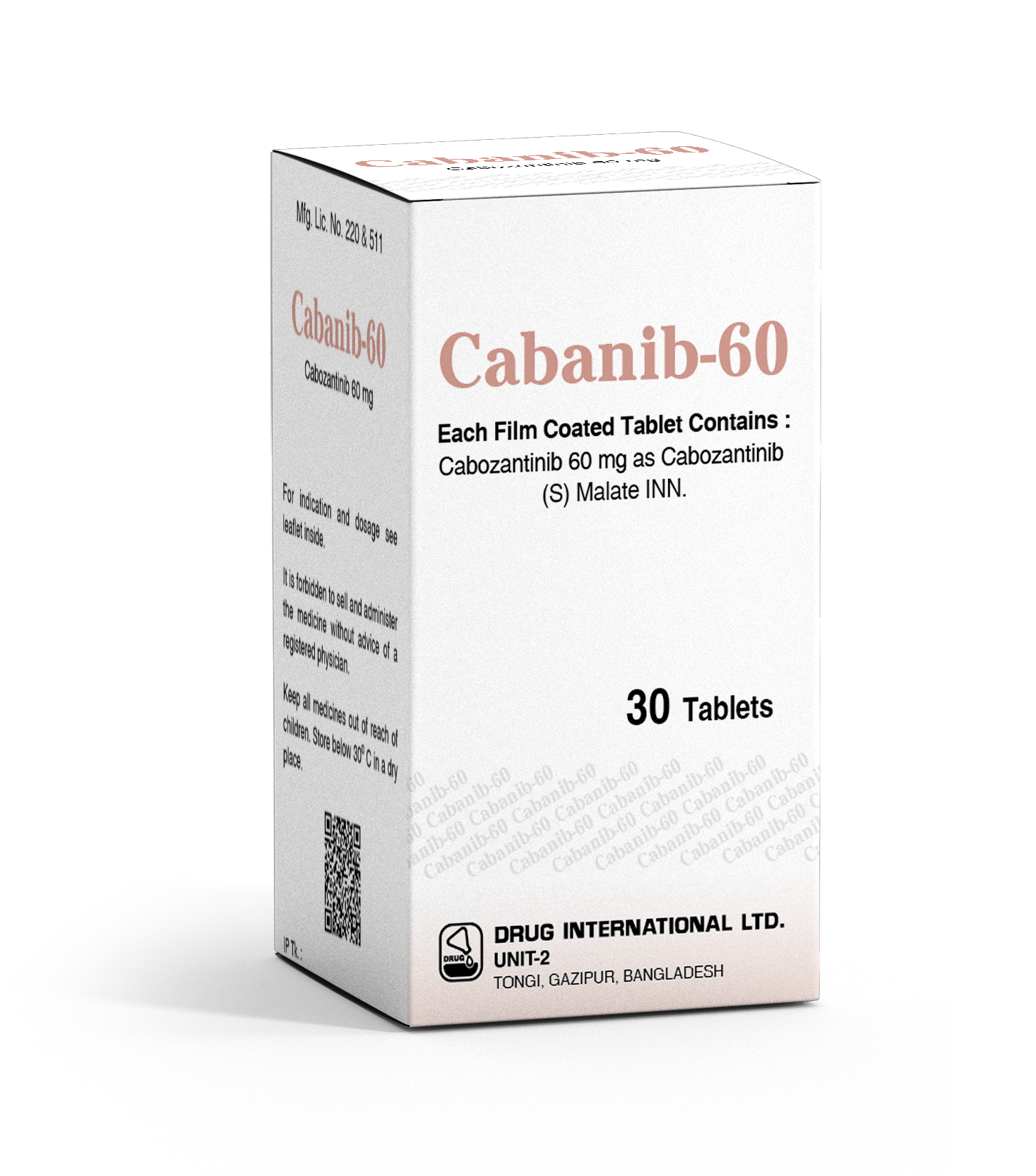
CABANIB(Cabozantinib)60Mg
£0.00
CABANIB capsules comprise 60mg of Cabozantinib as the active component. Cabozantinib is categorized as a tyrosine kinase inhibitor, predominantly employed in oncology to address diverse cancer types. Its mechanism involves directing particular enzymes crucial for cancer cell proliferation and dissemination. Healthcare providers prescribe CABANIB capsules as an integral component of treatment for qualifying patients with specific cancer types.
Indication:
CABANIB capsules, containing 60mg of Cabozantinib, are prescribed for treating various cancers.
Pharmacology:
Cabozantinib functions as a tyrosine kinase inhibitor, targeting specific enzymes crucial for cancer cell proliferation and spread. It blocks multiple receptor tyrosine kinases (RTKs), including MET, AXL, and VEGFR, pivotal in tumor growth, angiogenesis, and metastasis.
Dosage and Administration:
The typical dosage of CABANIB capsules is orally administered once daily, with or without food. Adjustments in dosage may be required based on individual patient factors and tolerability.
Interaction:
Cabozantinib might interact with certain medications, including strong CYP3A4 inhibitors and inducers. Patients must inform their healthcare provider about all medications, supplements, and herbal products they are taking to prevent potential interactions.
Side Effects:
Common side effects of CABANIB capsules may comprise fatigue, diarrhea, nausea, hypertension, and reduced appetite. More severe side effects like hepatotoxicity, hemorrhage, thromboembolism, and gastrointestinal perforation can also occur. Patients should promptly report any severe or persistent side effects to their healthcare provider.
Precautions and Warnings:
Patients undergoing treatment with CABANIB capsules should undergo regular monitoring for potential adverse reactions, including liver function tests and blood pressure checks. Cabozantinib usage during pregnancy may lead to fetal harm, necessitating contraception use by both males and females during treatment and afterward. Breastfeeding is discouraged while on CABANIB.
Overdose Effects:
In instances of overdose with CABANIB capsules, patients may experience intensified adverse effects such as hypertension, diarrhea, and fatigue. Management typically involves supportive care and monitoring for complications. Immediate medical attention is recommended if overdose is suspected.
| Product Name | CABANIB |
|---|---|
| Generic Name | Cabozantinib |
| Formulation | Capsule |
| Available Pack Size | 30's |
| Available Strength | 60Mg |
You must be logged in to post a review.


Reviews
There are no reviews yet.