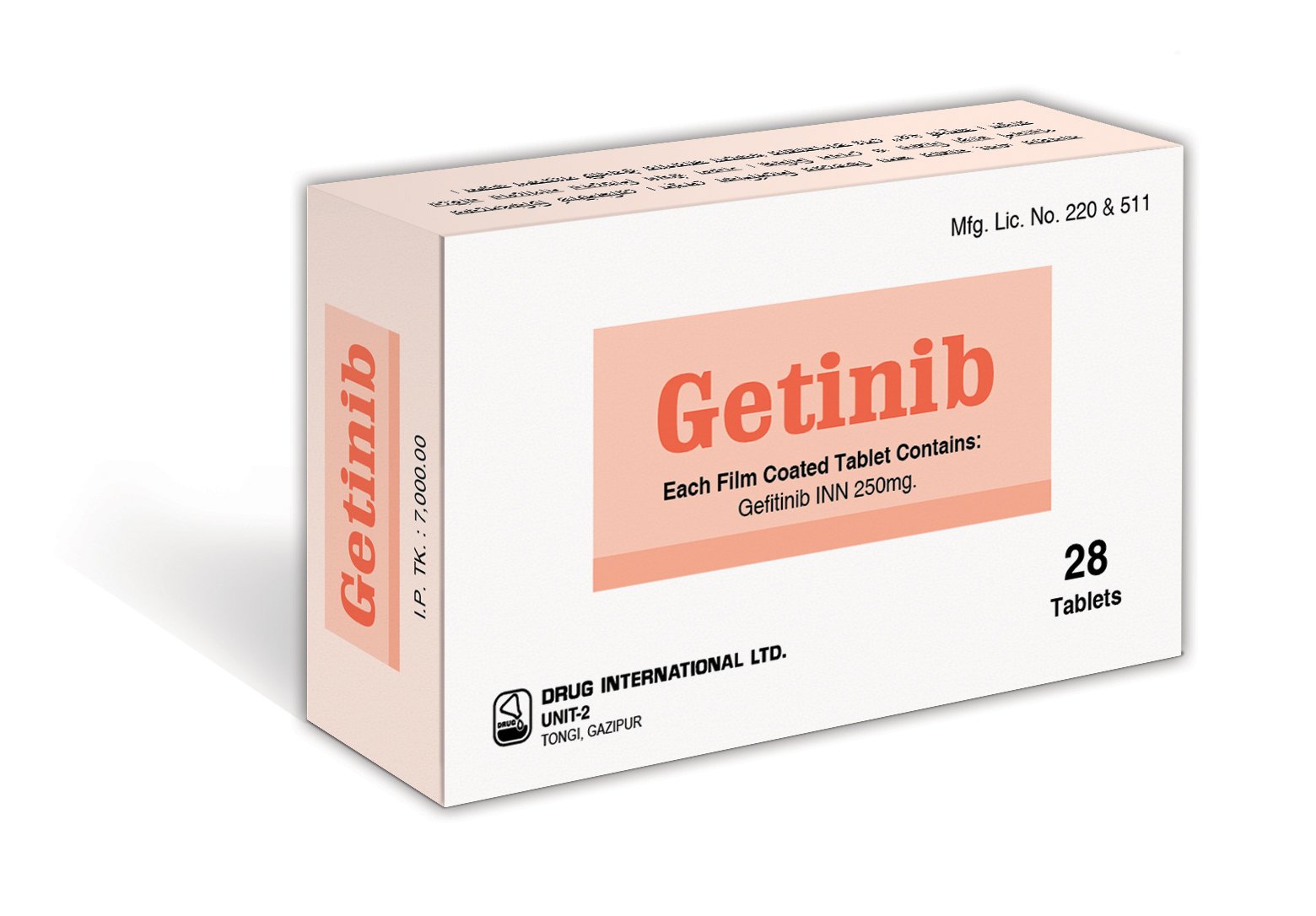
Getinib (Gefitinib) 250 mg
£0.00
Gefitinib is a medication classified as a tyrosine kinase inhibitor (TKI) that targets the epidermal growth factor receptor (EGFR). It is primarily used for the treatment of certain types of non-small cell lung cancer (NSCLC) that have specific EGFR mutations. By inhibiting EGFR, gefitinib helps to slow down the growth and spread of cancer cells. It is typically administered orally in tablet form and is available in strengths such as 250 mg. Common side effects may include rash, diarrhea, and liver problems, so it’s important for patients to be monitored closely by their healthcare provider while taking this medication.
Composition: Each film-coated tablet of Getinib contains 250 mg of Gefitinib.
Clinical Pharmacology: Gefitinib inhibits the kinase activity of EGFR, preventing autophosphorylation of tyrosine residues associated with the receptor. This action blocks downstream signaling and inhibits EGFR-dependent proliferation. Gefitinib has higher binding affinity for certain EGFR mutations compared to wild-type EGFR. It also inhibits signaling mediated by IGF and PDGF. Its metabolism primarily occurs in the liver through CYP3A4, with multiple identified biotransformation pathways. The major active metabolite is O-desmethyl Gefitinib.
Pharmacokinetics: Oral bioavailability is approximately 60%, with peak plasma levels reached 3-7 hours post-dose. Food does not significantly affect bioavailability. Gefitinib is extensively metabolized in the liver and cleared primarily via feces. The elimination half-life is around 48 hours, and steady-state concentrations are achieved within 10 days of daily dosing.
Indications: Getinib is indicated as first-line treatment for metastatic non-small cell lung cancer (NSCLC) with EGFR exon 19 deletions or exon 21 (L858R) substitution mutations.
Dosage and Administration: The recommended dose is 250 mg orally once daily with or without food until disease progression or unacceptable toxicity. Tablets can be dissolved in water for patients with difficulty swallowing solids. Dose modifications are recommended for specific adverse reactions, and treatment should be resumed upon resolution of adverse events.
Side Effects: Common side effects include interstitial lung disease, hepatotoxicity, gastrointestinal perforation, diarrhea, ocular disorders (including keratitis), and bullous and exfoliative skin disorders.
Contraindications: Gefitinib is contraindicated in patients with known hypersensitivity to Gefitinib or any of its components.
Use in Pregnancy and Lactation: There are limited data on the use of Gefitinib in pregnant women. Its use during pregnancy should be carefully considered, and patients should be informed of potential risks to the fetus or pregnancy loss.
| Product Name | Getinib |
|---|---|
| Generic Name: | Gefitinib |
| Formulation: | Oral tablets |
| Available Pack Size: | 28 tablets |
| Available Strength: Common strengths | 250 mg tablets |
You must be logged in to post a review.



Reviews
There are no reviews yet.