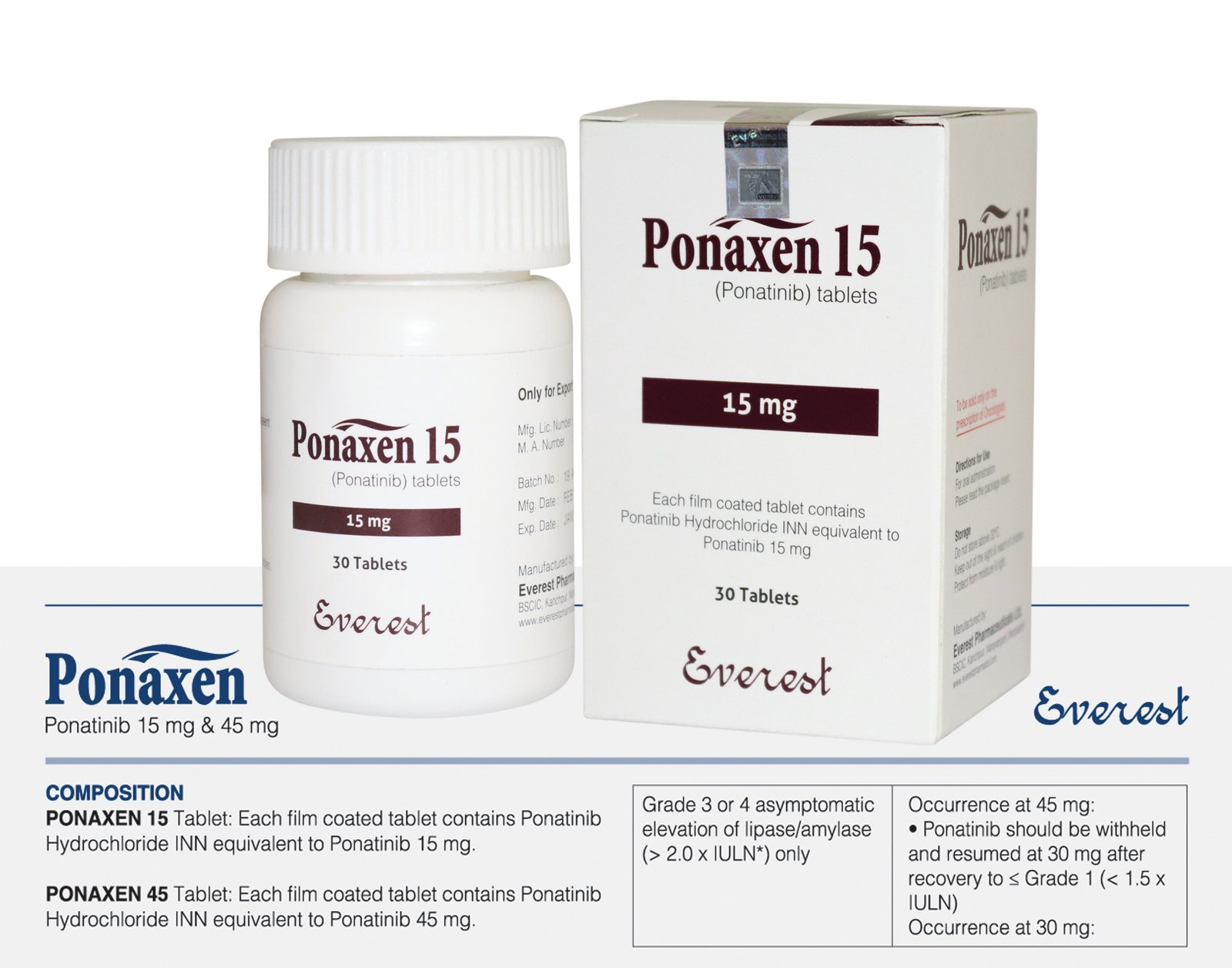
Ponaxen (Ponatinib Hydrochloride INN equivalent to Ponatinib) 15 mg
Ponaxen, containing Ponatinib Hydrochloride, which is the International Nonproprietary Name (INN) equivalent to Ponatinib, is prescribed for chronic myeloid leukemia (CML) and Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL) when prior treatments with tyrosine kinase inhibitors (TKIs) have failed or caused intolerance. As a TKI, Ponaxen targets BCR-ABL, a significant factor in CML and Ph+ ALL, hindering cell growth and prompting cell death, even in cases where resistance to other TKIs is present. The standard dosage is 15 mg orally once daily, with or without food, requiring careful monitoring due to potential drug interactions. Common adverse effects encompass arterial blood clotting, liver issues, and bone marrow suppression, while severe complications may involve heart failure and arterial blockages. In cases of overdose, supportive care is crucial, as specific antidotes are unavailable. Regular monitoring of liver and pancreatic enzymes is advised to address potential risks.
Indications: Ponaxen is prescribed for chronic myeloid leukemia (CML) and Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) in adults who haven’t responded well to previous tyrosine kinase inhibitor therapy.
Pharmacology: Ponaxen, a tyrosine kinase inhibitor, blocks several tyrosine kinases, including BCR-ABL, crucial in CML and Ph+ ALL. This inhibition disrupts signaling pathways essential for leukemia cell growth and survival.
Dosage and Administration: The standard Ponaxen dose is 15 mg once daily, typically taken with food. Adjustments may be necessary based on individual response and side effects.
Interactions: Ponaxen may interact with other medications, emphasizing the importance of disclosing all medications, including supplements, to healthcare providers.
Side Effects: Common side effects may include headache, fatigue, nausea, vomiting, diarrhea, abdominal pain, and skin rash. More severe effects could involve high blood pressure, blood clotting issues, heart problems, liver toxicity, and pancreatitis.
Precautions and Warnings: Ponaxen use may lead to liver toxicity, pancreatitis, and vascular occlusive events, warranting regular monitoring of liver function, lipase levels, and vascular signs. Patients with heart failure or fluid retention history should be closely monitored for fluid retention.
Overdose Effects: In instances of overdose, supportive measures are recommended. Specific antidotes aren’t available, so treatment focuses on addressing symptoms and providing supportive care.
| Product Name | Ponaxen |
|---|---|
| Generic Name | Ponatinib Hydrochloride INN equivalent to Ponatinib |
| Formulation | Tablet |
| Available Pack size | 30 Tablets |
| Strengths | 15 mg |
You must be logged in to post a review.



Reviews
There are no reviews yet.